Publication
526
J. Fluorine
Chem., 107 (2),
285-300, 2001
DOI: 10.1016/S0022-1139(00)00372-9 |
|
|
Electrochemically induced free-radical tandem
cyclisation of chlorodifluoromethylated
ketones. Application to the synthesis of gem-difluorinated
heterocycles.
|
Philippe Happiot and Maurice Médebielle
Contribution from the Laboratoire d'Electrochimie Moléculaire, Unité Mixte de Recherche Université -
CNRS No 7591, Université de Paris 7 - Denis Diderot, 2 place Jussieu, 75251 Paris Cedex 05, France
The synthesis of a series of chlorodifluoromethylated
ketones 1–6 is
presented and the cyclic voltammetry of the reductive
cleavage of these ketones was investigated, in N,N-dimethylformamide
(DMF), at an inert electrode. Indirect electrochemical
reduction (by means of an electrogenerated anion
radical) in acetonitrile (CH3CN) or
in N,N-dimethylformamide (DMF),
of the naphthalene-derived chlorodifluoroacetylated
compounds 1 and 2 in
the presence of the olefinic substrates 7–10,
yields new gem-difluoro heterocyclic compounds 11–16 after
intramolecular cyclisation of a 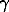 , ,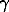 -difluoroalkyl radical. Aromatic nucleophilic substitution of -difluoroalkyl radical. Aromatic nucleophilic substitution of  , , -difluoroketones 12 and 13, in anhydrous
dimethylsulfoxide, with several tetramethylammonium
salts of imidazole as nucleophiles, proceeds
under mild conditions to give the corresponding
nitrogen–nitrogen exchanged products 17–23 in
moderate to good yields -difluoroketones 12 and 13, in anhydrous
dimethylsulfoxide, with several tetramethylammonium
salts of imidazole as nucleophiles, proceeds
under mild conditions to give the corresponding
nitrogen–nitrogen exchanged products 17–23 in
moderate to good yields |

