Publication
536
J. Am.
Chem. Soc., 123 ((27), 6669
-6677, 2001
DOI: 10.1021/ja0106063 S0002-7863(01)00606-0 |
|
|
Single Two-Electron Transfers vs Successive One-Electron Transfers
in Polyconjugated Systems Illustrated by the Electrochemical
Oxidation and Reduction of Carotenoids
|
Philippe Hapiot, Lowell D. Kispert, Valery V. Konovalov, and
Jean-Michel Savéant
Contribution from the Department of Chemistry, Box 870336, University of Alabama,
Tuscaloosa, Alabama 35487, and the Laboratoire d'Electrochimie Moléculaire,
Unité mixte de Recherche Université-CNRS No. 7591, Université de Paris 7 -Denis Diderot,
Case Courier 7107, 2 Place Jussieu, 75251 Paris Cedex 05, France
Examination of cyclic voltammetric responses reveals that inversion of the standard potentials of
the first and second electron transfers occurs in the oxidation of 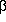 -carotene and 15,15'-didehydro- -carotene and 15,15'-didehydro-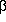 -carotene
(but not in their reduction) as well as in the reduction of canthaxanthin (but not in its oxidation). The factors
that control potential inversion in these systems, and more generally in symmetrical molecules containing
conjugated long chains, are investigated by quantum chemical calculations. Two main interconnected effects
emerge. One is the localization of the charges in the di-ion toward the ends of the molecule at a large distance
from one another, thus minimizing Coulombic repulsion. The same effect favors the solvation of the di-ion
providing additional stabilization. In contrast, the charge in the ion radical is delocalized over the whole molecular
framework, thus disfavoring its stabilization by interaction with the solvent. The combination of the two solvation
effects allows potential inversion to occur as opposed to the case where the two electrophores are linked by
a saturated bridge where potential inversion cannot occur. Localization of the charges in the di-ion, and thus
potential inversion, is favored by the presence of electron-accepting terminal groups for reductions (as the two
carbonyl groups in canthaxanthin) and of hole-accepting terminal groups for oxidations (as in -carotene
(but not in their reduction) as well as in the reduction of canthaxanthin (but not in its oxidation). The factors
that control potential inversion in these systems, and more generally in symmetrical molecules containing
conjugated long chains, are investigated by quantum chemical calculations. Two main interconnected effects
emerge. One is the localization of the charges in the di-ion toward the ends of the molecule at a large distance
from one another, thus minimizing Coulombic repulsion. The same effect favors the solvation of the di-ion
providing additional stabilization. In contrast, the charge in the ion radical is delocalized over the whole molecular
framework, thus disfavoring its stabilization by interaction with the solvent. The combination of the two solvation
effects allows potential inversion to occur as opposed to the case where the two electrophores are linked by
a saturated bridge where potential inversion cannot occur. Localization of the charges in the di-ion, and thus
potential inversion, is favored by the presence of electron-accepting terminal groups for reductions (as the two
carbonyl groups in canthaxanthin) and of hole-accepting terminal groups for oxidations (as in 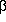 -carotene). -carotene).
|

