Publication
545
J. Fluorine
Chem., 109 (1), 39-48, 2001
DOI: 10.1016/S0022-1139(01)00378-5 |
|
|
Synthesis and reactivity of halogeno-difluoromethyl aromatics and
heterocycles. Application to the synthesis of gem-difluorinated bioactive
compounds
|
Conrad R. Burkholder,
William R. Dolbier, Jr. and
Maurice Médebielle
Contribution from the Department
of Chemistry, University of Florida, P.O. Box 117200, Gainesville, FL 32611-7200,
USA, and the Laboratoire d'Electrochimie Moléculaire, Université
Paris 7 Denis Diderot, UMR CNRS 7591, 2 Place Jussieu, 75251 Paris Cedex 05,
France
In an effort to prepare new fluorine-containing compounds which are active
against HIV, and based on the electrochemical reduction of a series of
bromodifluoromethyl compounds, the tetrakis(dimethylamino)ethylene (TDAE) was
found to be an effective reductant of the 2-(bromodifluoromethyl)benzoxazole
1 and of the 5-(bromodifluoromethyl)-3-phenyl-1,2,4-oxadiazole
3. A stepwise electron transfer with a difluoromethyl radical
as intermediate is assumed to take place in this reaction. Under mild
conditions, the generated difluoromethyl heterocyclic anion was efficiently
trapped with aromatic and heterocyclic aldehydes
7–14 and ketones
15–16. In this way the corresponding 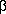 , ,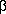 -difluoro- -difluoro- -heteroarylated alcohols 17–32 were
obtained in moderate to good yields. The same methodology was successfully
applied to the reduction of chlorodifluoromethylated ketones
4–6 and the generated -heteroarylated alcohols 17–32 were
obtained in moderate to good yields. The same methodology was successfully
applied to the reduction of chlorodifluoromethylated ketones
4–6 and the generated  , , -difluoroacetyl anion was trapped with several aldehydes
7, 8, 10,
11, under mild conditions, to give the corresponding
2,2-difluoro-3-hydroxy ketone derivatives
33–38, in moderate yields. The SRN1
reactions of 2-(bromodifluoromethyl)benzoxazole (1) with the
anions of heterocyclic thiols and phenolic compounds were also carried out. The
products 39–54, which all have a
CF2 group, were tested for activity against HIV, and several were
found to be active, including 44 which was very active. -difluoroacetyl anion was trapped with several aldehydes
7, 8, 10,
11, under mild conditions, to give the corresponding
2,2-difluoro-3-hydroxy ketone derivatives
33–38, in moderate yields. The SRN1
reactions of 2-(bromodifluoromethyl)benzoxazole (1) with the
anions of heterocyclic thiols and phenolic compounds were also carried out. The
products 39–54, which all have a
CF2 group, were tested for activity against HIV, and several were
found to be active, including 44 which was very active. |

