Publication
583
J.
Am. Chem. Soc., 126 (45), 14787-14795,
2004
DOI: 10.1021/ja046467h S0002-7863(04)06467-4 |
|
|
|
Why Are Proton Transfers at Carbon Slow? Self-Exchange
Reactions
|
|
Cyrille Costentin ,
and Jean-Michel Savéant
Contribution from the Laboratoire d'Electrochimie Moléculaire, Unité Mixte
de Recherche Université, CNRS No. 7591, Université de Paris 7, Denis Diderot, 2
place Jussieu, 75251 Paris Cedex 05, France
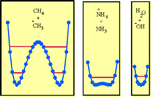
hen the quantum character of proton transfer is taken into account, the
intrinsic slowness of self-exchange proton transfer at carbon appears as a
result of its nonadiabatic character as opposed to the adiabatic character of
proton transfer at oxygen and nitrogen. This difference is caused by the lesser
polarity of C-H bonds as compared to that of O-H and N-H bonds. Besides solvent
and heavy-atom intramolecular reorganizations, the kinetics of the reaction are
consequently governed at the level of a pre-exponential term by proton tunneling
through the barrier. These contrasting behaviors are illustrated by an analysis
of the CH3H + -CH3, H2O +
OH-, and +NH4 + NH3 self-exchange
reactions. The effect of electron-withdrawing substituents and the case of
cation radicals are discussed within the same framework taking the
O2NCH2H + CH2=NO2- and
+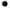 H2NCH2H
+ H2NCH2H
+ 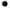 CH2NH2
as examples. Illustrated by the CH2=CH-CH2H +
-CH2-CH=CH2 couple, it is shown that the
"imbalanced character of the transition state" is related to heavy-atom
intramolecular reorganization. Combination of these various effects is finally
analyzed, taking the O2N-CH2=CH-CH2H +
CH2=CH-CH=NO2- and + CH2NH2
as examples. Illustrated by the CH2=CH-CH2H +
-CH2-CH=CH2 couple, it is shown that the
"imbalanced character of the transition state" is related to heavy-atom
intramolecular reorganization. Combination of these various effects is finally
analyzed, taking the O2N-CH2=CH-CH2H +
CH2=CH-CH=NO2- and +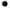 H2N-CH2=CH-CH2H
+ H2N-CH2=CH-CH2H
+  CH2-CH=CH2-NH2
couples as examples. CH2-CH=CH2-NH2
couples as examples. |

