Publication
585
J.
Am. Chem. Soc., 126 (51), 16834-16840,
2004
DOI: 10.1021/ja045294t S0002-7863(04)05294-1 |
|
|
Stepwise and Concerted Electron-Transfer/Bond Breaking
Reactions. Solvent Control of the Existence of Unstable  Ion Radicals and of the Activation Barriers of Their Heterolytic
Cleavage Ion Radicals and of the Activation Barriers of Their Heterolytic
Cleavage |
|
 |
|
Cyrille Costentin ,
Marc Robert and Jean-Michel Savéant*
Contribution from the Laboratoire d'Electrochimie Moléculaire, Unité Mixte
de Recherche Université, CNRS No. 7591, Université de Paris 7, Denis Diderot, 2
place Jussieu, 75251 Paris Cedex 05, France
*saveant
@laposte.net
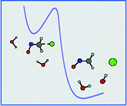
Available data from various sources seem to indicate an important role of
solvation in the cleavage rates of intermediate  ion radicals, in the passage from concerted to stepwise
electron-transfer/bond breaking reaction pathways and even in the very existence
of ion radicals, in the passage from concerted to stepwise
electron-transfer/bond breaking reaction pathways and even in the very existence
of  ion radicals. After preliminary computations treating the solvent as
dielectric continuum, these expectations are examined with the help of a simple
model system involving the anion radical of ONCH2Cl and two molecules
of water, which allows the application of advanced computational techniques and
a treatment of these solvent effects that emphasizes the role of solvent
molecules that sit close to the charge centers of the molecule. A ion radicals. After preliminary computations treating the solvent as
dielectric continuum, these expectations are examined with the help of a simple
model system involving the anion radical of ONCH2Cl and two molecules
of water, which allows the application of advanced computational techniques and
a treatment of these solvent effects that emphasizes the role of solvent
molecules that sit close to the charge centers of the molecule. A  ion radical minimum indeed appears upon introduction of the two water
molecules, and cleavage is accompanied by their displacement toward the leaving
anion, thus offering a qualitative mimicry of the experimental observations. ion radical minimum indeed appears upon introduction of the two water
molecules, and cleavage is accompanied by their displacement toward the leaving
anion, thus offering a qualitative mimicry of the experimental observations.
|

