Publication
590
Langmuir 21 (8), 3362 -3375, 2005
DOI: 10.1021/la047139y S0743-7463(04)07139-2
|
|
|
Dense Monolayers of Metal-Chelating Ligands Covalently
Attached to Carbon Electrodes Electrochemically and Their Useful Application in
Affinity Binding of Histidine-Tagged Proteins |
|
|
|
| |
 |
|
|
|
|
|
|
Ronald Blankespoor,
Benoît Limoges, Bernd Schöllhorn, Jean-Laurent Syssa-Magalé,
and Dounia Yazidi
Department of Chemistry, Calvin College, 3201 Burton SE, Grand Rapids,
Michigan 49546, Laboratoire d'Electrochimie Moléculaire de l'Université Denis
Diderot (Paris 7), UMR CNRS 7591, 2 place Jussieu, 75251 Paris Cedex 05, France,
and Département de Chimie, Ecole Normale Supérieure, UMR CNRS 8640 - PASTEUR, 24
rue Lhomond, 75231 Paris Cedex 05, France
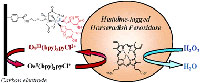
In this work, monolayers of metal complexes were covalently attached to the
surface of carbon electrodes with the goal of binding monolayers of
histidine-tagged proteins with a controlled molecular orientation and a
maintained biological activity. In this novel method, which is simple,
versatile, and efficient, the covalent attachment was accomplished in a single
step by the electrochemical reduction of aryl diazonium ions that were
substituted with a nitrilotriacetic (NTA) or an imminodiacetic (IDA) ligand at
the para position. The transient aryl radicals that were generated in the
reduction were grafted to the surfaces of glassy carbon, highly oriented
pyrolitic graphite, and graphite-based screen-printed electrodes, producing
dense monolayers of the ligands. The NTA- and IDA-modified electrodes were shown
to efficiently chelate Cu(II) and Ni(II) ions. The presence of the metal was
established using X-ray photoelectron spectroscopy and electrochemistry. Surface
coverages of the ligands were indirectly determined from the electroactivity of
the copper(II) complex formed on the electrode surface. Studies on the effect of
electrodeposition time and potential showed that, at sufficiently negative
potentials, the surface coverage reached a saturating value in less than 2 min
of electrodeposition time, which corresponds to the formation of a close-packed
monolayer of ligand on the electrode surface. Once loaded with a metal ion, the
modified electrode was able to bind specifically to histidine-tagged proteins
such as the horseradish peroxidase (His-HRP) or to an enhanced, recombinant
green-fluorescent protein via its N-terminal hexahistidine tail. In the case of
His-HRP, the amount of active enzyme specifically immobilized by metal-chelating
binding was determined from the analysis of electrocatalytic currents using
cyclic voltammetry. The electrochemical grafting makes it possible to accurately
controlled and electronically address the amount of deposited ligand on the
conductive surfaces of carbon electrodes with any size and shape. |

