Publication
593
J.
Am. Chem. Soc. 127 (35), 12154 -12155, 2005
DOI: 10.1021/ja0520464 S0002-7863(05)02046-9
|
|
|
Does Catalysis of Reductive Dechlorination of Tetra- and
Trichloroethylenes by Vitamin B12 and Corrinoid-Based Dehalogenases Follow an
Electron Transfer Mechanism?
|
|
|
|
| |
 |
|
|
|
|
|
|
Cyrille
Costentin, Marc Robert and Jean-Michel Savéant*
Laboratoire d'Electrochimie Moléculaire,
Université de Paris 7 - Denis
Diderot, Case Courrier 7107, 2 place Jussieu, 75251 Paris Cedex 05, France
Knowing the mechanism by which dangerous organic chloride pollutants, such as
tetra- or trichloroethylene, are reductively cleaved is an important task for
the establishment of remediation strategies and for a better comprehension of
bacterial dehalorespiration by corrinoid-based dehalogenases. On the basis of
electrochemical and thermodynamic data, application of outersphere and
dissociative electron transfer theories allows the prediction of the pertinent
activation/driving force relationships characterizing the electron transfer
mechanism. They are validated by application of the redox catalysis method to
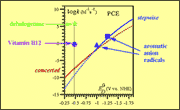
the reaction with two typical outersphere electron donors. The kinetic gap is
more than 11 and 7 orders of magnitude for the dehalogenase and for cobalamin,
respectively, showing that the electron transfer mechanism is not operative.
Multistep mechanisms in which the chloroethylene molecule enters the cobalt
coordination sphere are preferred. |

