Publication
595
J.
Am. Chem. Soc. 127 (39), 13583 -13588, 2005
DOI: 10.1021/ja053403d S0002-7863(05)03403-7
|
|
|
Electroenzymatic
reactions. Investigation
of a reductive dehalogenase
by means of electrogenerated
redox cosubstrates.
|
|
|
|
| |
 |
|
|
|
|
|
|
G. Dieckert, D. Gugova, B. Limoges, M. Robert, J-M. Savéant
Contribution from the Institut fur Mikrobiologie, FSU Jena, Philosphenweg 12, 07743 Jena, Germany, and Laboratoire d'Electrochimie Moléculaire, Université de Paris 7-Denis Diderot, 2 place Jussieu,75251 Paris Cedex 05, France
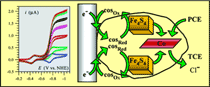
As an illustration of how cyclic voltammetry can be used to unravel the mechanisms and kinetics of redox enzymes, the reductive dechlorination of trichloroethylene and tetrachloroethylene by a typical reductive dehalogenase, the tetrachloroethene reductive dehalogenase of Sulfurospirillum multivorans (formerly called Dehalospirillum multivorans), was investigated by means of several electrochemically generated cosubstrates. They comprised the monocation and the neutral form of methylviologen, the neutral form of benzylviologen, and cobaltocene. Cyclic voltammetry is used to produce the active form of the cosubstrate under controlled potential conditions. It shows large plateau-shaped catalytic responses, which are used to measure the kinetics of the enzymatic reaction as a function of the substrate and cosubstrate concentrations. The variation of the rate constant for the cosubstrate reaction with its standard potential shows the transition between two asymptotic behaviors, one in which the reaction is under diffusion control and the other in which it is under counter-diffusion control. Simple fitting of this plot allows an estimation of the standard potential of the electron acceptor center in the enzyme (E = -0.57 V vs NHE). = -0.57 V vs NHE). |

