Publication
597
J.
Am. Chem. Soc. 128( 2 ), 2084-2092, 2006 .
DOI: 10.1021/ja0569196
|
|
|
Electrochemistry of Immobilized Redox Enzymes: Kinetic
Characteristics of NADH Oxidation Catalysis at Diaphorase Monolayers Affinity
Immobilized on Electrodes
|
|
|
|
|
 |
|
|
|
|
|
|
|
|
|
Benoît Limoges,* Damien Marchal, François Mavré, and Jean-Michel Savéant*
Contribution from the Laboratoire d'Electrochimie Moléculaire, Université de Paris 7-Denis Diderot, 2 place Jussieu,75251 Paris Cedex 05, France
In the class of NADH:acceptor oxidoreductases, the diaphorase from
Bacillus stearothermophilus is a particularly promising enzyme for
sensing NADH, and indirectly a great number of analytes, when coupled with a
NAD-dependent dehydrogenase as well as for the design of mono- and multienzyme
affinity sensors. The design and rational optimization of such systems require
devising immobilization procedures that prevent dramatic losses of the enzymatic
activity and a full kinetic characterization of the immobilized enzyme system.
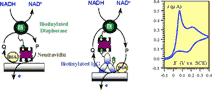
Two immobilization procedures are described, which involve recognition of the
biotinylated diaphorase by a monolayer of neutravidin adsorbed on the electrode
surface either directly or through the intermediacy of a monolayer of
biotinylated rabbit immunoglobulin. Thorough kinetic characterization of the two
systems is derived from cyclic voltammetric responses. A precise estimate of the
enzyme coverages is obtained after comparing the enzyme kinetics of the
immobilized and the homogeneous system. . |

