Publication
599
J. Am. Chem. Soc., ,128, 4552 -4553, 2006 .
DOI: 10.1021/ja060527x
|
|
|
Electrochemical and Homogeneous Proton-Coupled Electron
Transfers: Concerted Pathways in the One-Electron Oxidation of a Phenol Coupled
with an Intramolecular Amine-Driven Proton Transfer
|
|
|
|
|
 |
|
|
|
|
|
|
|
|
|
Cyrille Costentin, Marc Robert, and Jean-Michel Savéant*
Contribution from the Laboratoire d'Electrochimie Moléculaire, Université de Paris 7-Denis Diderot, 2 place Jussieu,75251 Paris Cedex 05, France
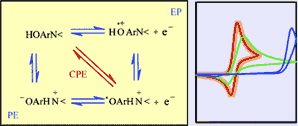
Proton-coupled electron transfers currently attract considerable attention in
view of their likely involvement in many natural processes. Electrochemistry,
through techniques such as cyclic voltammetry, is an efficient way of
investigating the reaction mechanism of these reactions, and deciding whether
proton and electron transfers are concerted or occur in a stepwise manner. The
oxidation of an ortho-substituted 4,6-di (tert-butyl)-phenol in which the
phenolic hydrogen atom is transferred during the reaction to the nitrogen atom
of a nearby amine is taken as illustrative example. A careful analysis of the
cyclic voltammetric responses obtained with this compound and its OD derivative
allows, after estimation of the various thermodynamic parameters, ruling out the
occurrence of the square scheme mechanism involving the proton-electron and
electron-proton sequences. Simulation and comparison of the rate constant and
H/D kinetic isotope effect with theoretical predictions show that the
experimental value of the preexponential factor is ca. 1 order of magnitude
larger than the theoretical value. Detailed calculations suggest that an
electric field effect is responsible for this discrepancy. |

