Publication
615
J. Am. Chem. Soc. , 129,
5870 - 5879
, 2007.
DOI:
10.1021/ja067950q
|
|
|
|
|
|
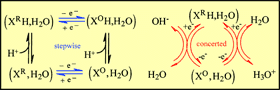 |
Concerted Proton-Electron Transfer Reactions in Water. Are the Driving Force and Rate Constant Depending on pH When Water Acts as Proton Donor or Acceptor? |
|
|
|
|
|
|
|
Cyrille Costentin, Marc Robert, and Jean-Michel Savéant
Contribution from the Laboratoire d'Electrochimie Moléculaire, Unité Mixte de Recherche Université, CNRS No 7591, Université de Paris 7-Denis Diderot, 2 place Jussieu, 75251 Paris Cedex 05, France
The competition between stepwise and concerted (CPET) pathways in proton-coupled electron-transfer reactions in water is discussed on thermodynamic and kinetic bases. In the case where water is
the proton acceptor, the CPET pathway may compete favorably with the stepwise pathway. The main
parameter of the competition is pK of the oxidized form of the substrate being smaller or larger than 0. The
driving force of the forward reaction is however independent of pH, despite the equilibrium redox potential
of the proton-electron system being a function of pH. At high pH values, CPET reactions involving OH-
as proton acceptor may likewise compete favorably with stepwise pathways. The overall reaction rate
constant is an increasing function of pH, not because the driving force depends on pH but because OH-
is a reactant. In buffered media, association of the substrate with the basic components of the buffer offers
an alternative CPET route; the driving force comes closer to that offered by the pH-dependent equilibrium
redox potential. |

