Publication
622
J. Phys. Chem. C 111, 12877-12880, 2007.
DOI:
10.1021/jp0750654
|
|
|
|
|
|
 |
Acceleration of the Homogeneous and Electrochemical Reductions of Dioxygen in Aprotic Media by Ammonium Ions. Is the Driving Force a Function of NH4+ Concentration? What Is the Mechanism of the Reaction? |
|
|
|
|
|
|
|
Cyrille Costentin, Marc Robert, and Jean-Michel Savéant
Contribution from the Laboratoire d'Electrochimie Moléculaire, Unité Mixte de Recherche Université, CNRS No 7591, Université de Paris 7-Denis Diderot, 2 place Jussieu, 75251 Paris Cedex 05, France
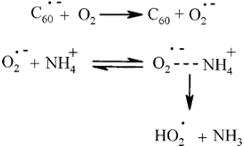
Previous interpretation of homogeneous reduction of dioxygen by the anion radical of C60 in aprotic medium
in the presence of NH4+, which is based on an electron-transfer driving force depending on NH4+ concentration
is not tenable because, from first principles, the driving force does not depend on reactant concentrations.
Data have been interpreted in a framework of an electron-proton transfer mechanism. An examination of the
electrochemical reduction of dioxygen in the presence of NH4+ shows that the mechanism is stepwise and
allows the determination of key thermodynamic and kinetic parameters. With C60 - as a homogeneous electron
donor, in the framework of the same stepwise mechanism, electron transfer acts as a pre-equilibrium while
protonation by NH4+ is rate-determining. In all cases, a second electron transfer, which requires less energy
than the first, leads after protonation by NH4+ to hydrogen peroxide. - as a homogeneous electron
donor, in the framework of the same stepwise mechanism, electron transfer acts as a pre-equilibrium while
protonation by NH4+ is rate-determining. In all cases, a second electron transfer, which requires less energy
than the first, leads after protonation by NH4+ to hydrogen peroxide. |

