Publication
636
Chem. Eur. J. 14, 9286-9291, 2008.
DOI:
10.1002/chem.200801168
|
|
|
|
|
|
 |
Electrochemical functionalization of carbon surfaces by aromatic azide or alkyne molecules: a versatile platform for click chemistry
|
|
|
|
|
|
|
|
David Evrard, François Lambert, Clotilde Policar, Véronique Balland, Benoît Limoges
Laboratoire de Thermodynamique et Interactions Moléculaires, Université Blaise Pascal, Aubière, France, and
Equipe de Chimie Bioorganique et Bioinorganique, ICMMO, UMR CNRS 8182, Université Paris-Sud 11, Bât. 420, 91405 Orsay, France
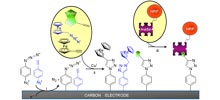
The electrochemical reduction of phenylazide or phenylacetylene diazonium salts leads to the grafting of azido or ethynyl groups onto the surface of carbon electrodes. In the presence of copper(I) catalyst, these azide- or alkyne-modified surfaces react efficiently and rapidly with compounds bearing an acetylene or azide function, thus forming a covalent 1,2,3-triazole linkage by means of click chemistry. This was illustrated with the surface coupling of ferrocenes functionalized with an ethynyl or azido group and the biomolecule biotin terminated by an acetylene group. |

