Publication
639
Chem. Eur. J.
15, 785-792, 2009.
DOI:
10.1002/chem.200801240
|
|
|
|
|
|
 |
Passage from Stepwise to Concerted Dissociative Electron Transfer through
Modulation of Electronic States Coupling. |
|
|
|
|
|
|
|
Cyrille Costentin, Ludovic Donati, and Marc Robert
Contribution from the Laboratoire d'Electrochimie Moléculaire, Unité Mixte de Recherche Université - CNRS No 7591, Université Paris - Diderot, Bätiment Lavoisier, 15 rue Jean de Baïf, 75205 Paris Cedex 13, France
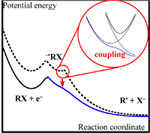
Reductive cleavage of the three cyanobenzyl chloride isomers in N,N-dimethylformamide gives new insights into the factors that control the mechanism during dissociative electron transfer. Within the family of investigated compounds, electrochemical reduction leads to expulsion of the chloride ion. While electron transfer is concerted with breaking of the C--Cl bond and acts as the rate-determining step in the case of both the ortho and para isomers, an intermediate anion radical is formed before rapid fragmentation in the case of the meta isomer. Such an unexpected mechanistic shift (all key thermodynamic parameters are very similar for the three chlorides) is interpreted in the framework of a modified version of the dissociative electron-transfer model that includes electronic coupling effects between the diabatic states of the products. These effects appear to control the very existence of a transient species along the reaction pathway. |

