Publication
658
J. Biol. Chem.
285, 7233–7245, 2010.
DOI: 10.1074/jbc.M109.038240
|
|
|
|
|
 |
Role of Arginine Guanidinium Moiety in Nitric-oxide Synthase Mechanism of Oxygen Activation |
|
|
|
|
|
Claire Giroud, Magali Moreau, Tony A. Mattioli, Véronique Balland, Jean-Luc Boucher, Yun Xu-Li, Dennis J. Stuehr and Jérôme Santolini
From the Institut de Biologie et de Technologies de Saclay, Laboratoire Stress Oxydants et Detoxication, Commissariat à l'Energie Atomique Saclay, 91191 Gif-sur-Yvette Cedex, France,
UMR 8601 CNRS, University Paris Descartes, 45 rue des Saints Peres, 75270 Paris, France,
the ?Lerner Research Foundation, Cleveland Clinic, Cleveland, Ohio 44195, and
the Laboratoire d'Electrochimie Moléculaire, University Paris Diderot, UMR 7591, 15 rue J.-A. de Baïf, 75205 Paris Cedex 13, France
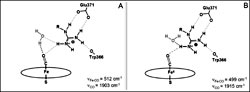
Nitric-oxide synthases (NOS) are highly regulated heme-thiolate enzymes that catalyze two oxidation reactions that sequentially
convert the substrate l-Arg first to N?-hydroxyl-l-arginine and then to l-citrulline and nitric oxide. Despite numerous investigations, the detailed molecular mechanism of NOS remains elusive and
debatable. Much of the dispute in the various proposed mechanisms resides in the uncertainty concerning the number and sources
of proton transfers. Although specific protonation events are key features in determining the specificity and efficiency of
the two catalytic steps, little is known about the role and properties of protons from the substrate, cofactors, and H-bond
network in the vicinity of the heme active site. In this study, we have investigated the role of the acidic proton from the
l-Arg guanidinium moiety on the stability and reactivity of the ferrous heme-oxy complex intermediate by exploiting a series
of l-Arg analogues exhibiting a wide range of guanidinium pKa values. Using electrochemical and vibrational spectroscopic techniques, we have analyzed the effects of the analogues on
the heme, including characteristics of its proximal ligand, heme conformation, redox potential, and electrostatic properties
of its distal environment. Our results indicate that the substrate guanidinium pKa value significantly affects the H-bond network near the heme distal pocket. Our results lead us to propose a new structural
model where the properties of the guanidinium moiety finely control the proton transfer events in NOS and tune its oxidative
chemistry. This model may account for the discrepancies found in previously proposed mechanisms of NOS oxidation processes.
|

