Publication
661
Langmuir
26 (12),10347–10356, 2010.
DOI: 10.1021/la100397g
|
|
|
|
|
 |
Cleavage-Sensing Redox Peptide Monolayers for the Rapid Measurement of the Proteolytic Activity of Trypsin and α-Thrombin Enzymes |
|
|
|
|
Jocelyne Adjémian‡, Agnès Anne*†, Gilles Cauet‡ and Christophe Demaille*†
† Laboratoire d'Electrochimie Moléculaire, Unité Mixte de Recherche Université - CNRS No. 7591, Université Paris Diderot -Paris 7, 15 rue Jean-Antoine de Baïf, 75205 Paris Cedex 13, France
‡ HORIBA Medical Parc Euromédecine, rue du Caducée, BP7290 34184 Montpellier Cedex 4, France
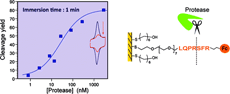
Ferrocene (Fc)-labeled peptides are end-grafted onto gold electrodes via a flexible polyethylene glycol (PEG) linker, and their ability to act as substrates for proteolytic enzymes trypsin and α-thrombin is investigated by cyclic voltammetry. It is shown that whereas a short Fc-tetrapeptide substrate is rapidly cleaved by trypsin, a longer Fc-heptapeptide substrate is required for α-thrombin detection. However, in both cases it is observed that not all of the Fc-peptide chains present on the electrode surface are cleavable by the proteases and that the cleavage yield is actually controlled by the surface coverage in the Fc-peptide. Surface dilution of the Fc-peptide using a backfilling molecule such as MCH (6-mercapto-1-hexanol) was required to obtain a cleavage yield larger than 80%. The kinetics of Fc-peptide cleavage by trypsin or α-thrombin is then shown to be adequately described by Michaelis Menten kinetics, allowing enzymatic constants kcat and KM to be determined. The obtained rate constant values showed that the affinity of the enzymes for their respective Fc-peptide substrates is very high (i.e., low KM values) whereas that for the cleavage step itself is relatively low (low kcat values). Partial compensation of these parameters yields a fast response of the Fc-peptide electrodes to the proteases in solution in the 1−1000 nM range. The type of molecule used to backfill the Fc-peptide layers, either MCH or PEG6 chains, is shown to modulate the activity of the proteases versus the Fc-peptide layers: in particular, the PEG6 diluent is specifically shown to decrease the ability of α-thrombin to cleave its Fc-peptide substrate whereas trypsin activity is unaffected by the presence of PEG chains. |

