Publication
670
PNAS, 107 (40),17113 - 17118, 2010
DOI: 10.1073/pnas.1011315107
|
|
|
|
|
 |
Electrochemical and homogeneous electron transfers to the alzheimer amyloid- β copper complex follow a preorganization mechanism |
|
|
|
|
Véronique Balland, Christelle Hureau, and Jean-Michel Savéant
Laboratoire d’Electrochimie Moléculaire, Unité Mixte de Recherche Université—Centre National de la Recherche Scientifique No. 7591, Université Paris Diderot, 15 rue Jean-Antoine de Baïf, 75205 Paris Cedex 13, France and Laboratoire de Chimie de Coordination, Unité Propre de Recherche Centre National de la Recherche Scientifique No. 8241, 205 Route de Narbonne, 31077 Toulouse Cedex, France
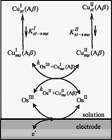
Deciphering the electron transfer reactivity characteristics of amyloid β-peptide copper complexes is an important task in connection with the role they are assumed to play in Alzheimer’s disease. A systematic analysis of this question with the example of the amyloid β-peptide copper complex by means of its electrochemical current–potential responses and of its homogenous reactions with electrogenerated fast electron exchanging osmium complexes revealed a quite peculiar mechanism: The reaction proceeds through a small fraction of the complex molecules in which the peptide complex is “preorganized” so as the distances and angles in the coordination sphere to vary minimally upon electron transfer, thus involving a remarkably small reorganization energy (0.3 eV). This preorganization mechanism and its consequences on the reactivity should be taken into account for reactions involving dioxygen and hydrogen peroxide that are considered to be important in Alzheimer’s disease through the production of harmful reactive oxygen species. |

