Publication
674
Anal. Chem.
82 (21),
8766– 8774, 2010.
DOI: 10.1021/ac101262v
|
|
|
|
|
|
 |
Bipolar Electrodes: A Useful Tool for Concentration, Separation, and Detection of Analytes in Microelectrochemical Systems |
|
|
|
|
|
|
|
François Mavré, Robbyn K. Anand, Derek R. Laws, Kwok-Fan Chow, Byoung-Yong Chang, John A. Crooks, and Richard M. Crooks
Université Paris Diderot (France), The University of Texas at Austin
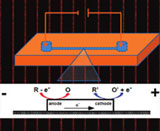
A bipolar electrode (BPE) is an electronic conductor in contact with an ionically conductive phase. When a sufficiently high electric field is applied across the ionic phase, faradaic reactions occur at the ends of the BPE even though there is no direct electrical connection between it and an external power supply. In this article, we describe the fundamental principles and some electroanalytical applications of BPEs for array-based sensing, separations, and concentration enrichment in microelectrochemical systems. Specifically, we show how the latter three operations, which are normally thought of as arising from different phenomena, are linked by processes occurring on and near BPEs confined within a convenient, miniaturized microfluidic format. The results presented here demonstrate that under a particular set of conditions, up to 1000 well-defined BPEs can be simultaneously activated and interrogated using just a single pair of driving electrodes. Furthermore, a slight change to the resistance of the buffer solution within the microfluidic channel leads to the separation and concentration enrichment of charged analytes. |

