Publication
694
J. Phys. Chem. B 115 (40), 11678-1168, 2011
DOI:10.1021/jp204615e
|
|
|
|
|
 |
Geometric and Electronic Structures of Peroxomanganese(III) Complexes Supported by Pentadentate Amino-Pyridine and -Imidazole Ligands |
|
|
|
|
Gaelle Filippini, Yael Israeli, Florent Goujon, Benoit Limoges, Christine Bonal, and Patrice Malfreyt
Laboratoire de Thermodynamique et Interactions Moléculaires, UMR CNRS 6272, Université Blaise Pascal, 63177 Aubière Cedex, France
Laboratoire de Photochimie Moléculaire et Macromoléculaire, UMR CNRS 6505, Université Blaise Pascal, 63177 Aubière Cedex, France
Laboratoire d’Electrochimie Moléculaire, UMR 7591 CNRS, Université Paris Diderot, Sorbonne Paris Cité, 15 rue Jean-Antoine de Baïf, F-75205 Paris Cedex 13, France
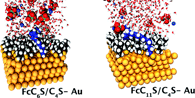
The free energy approach is used to study the effect of the relative chain length of the two constituents of electroactive self-assembled monolayers (SAMs) on gold. In this study, the ferrocene groups are exposed to the electrolyte solution. This situation is achieved by using shorter diluent alkanethiol chains. To this end, the mixed monolayers formed by the self-assembly of 11-ferrocenylundecanethiol and butanethiol FcC11S/C4S and of 6-ferrocenylhexanethiol and butanethiol FcC6S/C4S onto a gold surface are studied. Calculation of enthalpy and entropy differences are also performed using molecular simulations. Additionally, the electrochemical signatures of these systems are determined to allow a direct comparison with our calculations. The thermodynamic properties are discussed in terms of enthalpy and entropy changes. Two effects account for the thermodynamic behavior. The first one involves the ion pairing between the ferrocenium group and the perchlorate anion. The second one concerns the desolvation of the first hydration shell of the anions. Finally, this work is also completed with a microscopic description associated with an energy characterization of these SAMs as a function of the surface coverage under conditions close to experiments. |

