Publication
696
J. Am. Chem. Soc. 133 (47), 19160-19167, 2011
DOI:10.1021/ja206561n
|
|
|
|
|
 |
Concerted Proton–Electron Transfers. Consistency between Electrochemical Kinetics and their Homogeneous Counterparts |
|
|
|
|
Cyrille Costentin, Viviane Hajj, Cyril Louault, Marc Robert, and Jean-Michel Savéant
Univ Paris Diderot, Sorbonne Paris Cité, Laboratoire d'Electrochimie Moléculaire, Unité Mixte de Recherche Univ - CNRS No 7591, Bâtiment Lavoisier, 15 rue Jean de Baïf, 75205 Paris Cedex 13, France
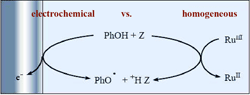
The concerted proton–electron transfer (CPET) oxidation of phenol with water (in water) and hydrogen phosphate as proton acceptors provides a good example for testing the consistency of the electrochemical and homogeneous approaches to a reaction, the comprehension of which raises more mechanistic and kinetic challenges than that of a simple outer-sphere electron transfer. Comparison of the intrinsic kinetic characteristics (obtained at zero driving force of the CPET reaction) shows that consistency is indeed observed after a careful identification and quantitation of side factors (electrical work terms, image force effects). Water (in water) appears as a better intrinsic proton acceptor than hydrogen phosphate in both cases in terms of reorganization energy and pre-exponential factor, corroborating the mechanism by which electron transfer is concerted with Grotthus-type proton translocation in water. Detailed compared analysis of the approaches also revealed that modest but significant electric field effects may be at work in the electrochemical case. Comparison with phenoxide ion oxidation, taken as a reference outer-sphere electron transfer, points to a CPET precursor complex that possesses a precise spatial structure allowing the formation of one or several H-bonds as required by the occurrence of the CPET reaction, thus decreasing considerably the number of efficient collisions compared with those undergone by structureless spherical reactants. |

