Publication
698
Phys. Chem. Chem. Phys. 14, 1581-1584, 2012
DOI:10.1039/C2CP23050J
|
|
|
|
|
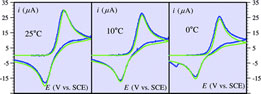 |
Reorganization energy and pre-exponential factor from temperature-dependent experiments in electron transfer reactions. A typical example: the reduction of tert-nitrobutane |
|
|
|
|
Cyrille Costentin, Cyril Louault, Marc Robert, Vincent Rogé and Jean-Michel Savéant
Univ Paris Diderot, Sorbonne Paris Cité, Laboratoire d'Electrochimie Moléculaire, Unité Mixte de Recherche Univ - CNRS No 7591, Bâtiment Lavoisier, 15 rue Jean de Baïf, 75205 Paris Cedex 13, France
The electrochemical one-electron reduction of tert-nitrobutane in N,N´-dimethylformamide is a typical reaction, which has been shown to follow the quadratic model of outersphere electron transfer. The variation of the standard rate constant with temperature allows a separate determination of the reorganization energy and of the pre-exponential factor. The value found for the former is in agreement with independent estimates of the solvent and intramolecular reorganization energies. The value of the latter, significantly larger than the collision frequency, implies that the reaction starts to take place before close contact with the electrode surface. |

