Publication
702
Inorg. Chem., 51 (6), 3603-3612, 2012
DOI: 10.1021/ic202480h
|
|
|
|
|
 |
Hydroxide Ion versus Chloride and Methoxide as an Exogenous Ligand Reveals the Influence of Hydrogen Bonding with Second-Sphere Coordination Water Molecules in the Electron Transfer Kinetics of Mn Complexes |
|
|
|
|
Sanae El Ghachtouli, Régis Guillot, Ally Aukauloo, Pierre Dorlet, Elodie Anxolabéhère-Mallart, and Cyrille Costentin
Laboratoire d’Electrochimie Moléculaire, Univ Paris Diderot, Sorbonne Paris Cité, Unité Mixte de Recherche Université−CNRS No. 7591, Bâtiment Lavoisier, 15 rue Jean de Baïf, 75205 Paris Cedex 13, France
ICMMO−UMR 8182−Bât. 420, Université Paris−Sud 11, 15 rue Georges Clemenceau, 91405 Orsay Cedex, France
Laboratoire Stress Oxydant et Détoxication, CNRS, UMR 8221, F-91191 Gif-sur-Yvette, France
CEA, iBiTec-S, SB2SM, F-91191 Gif-sur-Yvette, France
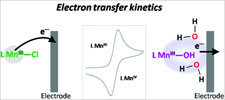
We recently reported on the synthesis of a new pentadentate N4O ligand, tBuL–, together with the X-ray diffraction structure of the corresponding mononuclear manganese(III)-hydroxo complex namely [(tBuL)Mn(III)OH](ClO4), (1 (ClO4)). [El Ghachtouli et al. Energy Environ. Sci. 2011, 4, 2041.] In the present work, we evidence through electrochemical analysis that complex 1+, in the presence of water, shows a peculiar behavior toward electron-transfer kinetics. The synthesis, single-crystal X-ray diffraction, and EPR spectroscopic characterization of two other mononuclear manganese(III)-chlorido and methoxo complexes—namely, [(tBuL)Mn(III)Cl](PF6), (2(PF6)) and [(tBuL)Mn(III)OMe](ClO4), (3(ClO4))—are also reported. 2(PF6) and 3(ClO4) compounds will serve as reference complexes for the electron-transfer kinetics investigation. The peculiar behavior of 1(ClO4) is attributed to the specificity of hydroxide anion as ligand presumably allowing intermolecular hydrogen-bonding interactions and thus affecting electron-transfer properties. |

