Publication
708
Langmuir, 28 (23), 8804-8813, 2012
DOI:10.1021/la301316r
|
|
|
|
|
 |
Optimizing Electrode-Attached Redox-Peptide Systems for Kinetic Characterization of Protease Action on Immobilized Substrates. Observation of Dissimilar Behavior of Trypsin and Thrombin Enzymes |
|
|
|
|
Agnès Anne, Arnaud Chovin, and Christophe Demaille
Laboratoire d’Electrochimie Moléculaire, UMR 7591 CNRS, Univ Paris Diderot, Sorbonne Paris Cité, 15 rue Jean-Antoine de Baïf, F-75205 Paris Cedex 13, France
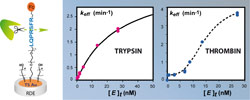
In this work, we experimentally address the issue of optimizing gold electrode attached ferrocene (Fc)-peptide systems for kinetic measurements of protease action. Considering human a-thrombin and bovine trypsin as proteases of interest, we show that the recurring problem of incomplete cleavage of the peptide layer by these enzymes can be solved by using ultraflat template-stripped gold, instead of polished polycrystalline gold, as the Fc-peptide bearing electrode material. We describe how these fragile surfaces can be mounted in a rotating disk configuration so that enzyme mass transfer no longer limits the overall measured cleavage kinetics. Finally, we demonstrate that, once the system has been optimized, in situ real-time cyclic voltammetry monitoring of the protease action can yield high-quality kinetic data, showing no sign of interfering effects. The cleavage progress curves then closely match the Langmuirian variation expected for a kinetically controlled surface process. Global fit of the progress curves yield accurate values of the peptide cleavage rate for both trypsin and thrombin. It is shown that, whereas trypsin action on the surface-attached peptide closely follows Michaelis–Menten kinetics, thrombin displays a specific and unexpected behavior characterized by a nearly enzyme-concentration-independent cleavage rate in the subnanomolar enzyme concentration range. The reason for this behavior has still to be clarified, but its occurrence may limit the sensitivity of thrombin sensors based on Fc-peptide layers. |

