Publication
713
Science , 338 (6103), 90-94, 5 October 2012
DOI:10.1126/science.1224581
|
|
|
|
|
 |
A Local Proton Source Enhances CO2 Electroreduction to CO by a Molecular Fe Catalyst |
|
|
|
|
Cyrille Costentin, Samuel Drouet, Marc Robert, and Jean-Michel Savéant
Université Paris Diderot, Sorbonne Paris Cité, Laboratoire d'Electrochimie Moléculaire, Unité Mixte de Recherche Université–CNRS no. 7591, Bâtiment Lavoisier, 15 Rue Jean de Baïf, 75205 Paris Cedex 13, France
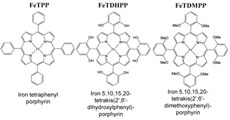
Electrochemical conversion of carbon dioxide (CO2) to carbon monoxide (CO) is a potentially useful step in the desirable transformation of the greenhouse gas to fuels and commodity chemicals. We have found that modification of iron tetraphenylporphyrin through the introduction of phenolic groups in all ortho and ortho’ positions of the phenyl groups considerably speeds up catalysis of this reaction by the electrogenerated iron(0) complex. The catalyst, which uses one of the most earth-abundant metals, manifests a CO faradaic yield above 90% through 50 million turnovers over 4 hours of electrolysis at low overpotential (0.465 volt), with no observed degradation. The basis for the enhanced activity appears to be the high local concentration of protons associated with the phenolic hydroxyl substituents.
-------------------------------------------------------------------------------------------------------------------------------------------------------------------
 Lire le Communiqué "En direct des laboratoires " de l'Institut de Chimie du CNRS : Lire le Communiqué "En direct des laboratoires " de l'Institut de Chimie du CNRS :
“Du fer pour transformer CO2 en CO, précurseur de carburant liquide synthétique” [05-10-2012] |

