Publication
715
Langmuir, 28 (39),14065-14072, 2012
DOI:10.1021/la302913j
|
|
|
|
|
 |
Spectroelectrochemical Characterization of Small Hemoproteins Adsorbed within Nanostructured Mesoporous ITO Electrodes |
|
|
|
|
Delphine Schaming, Christophe Renault, Ryan T. Tucker, Stéphanie Lau-Truong, Jean Aubard, Michael J. Brett, Véronique Balland, Benoît Limoges
Laboratoire d’Electrochimie Moléculaire, UMR CNRS 7591 and Laboratoire ITODYS, UMR CNRS 7086, Université Paris Diderot, Sorbonne Paris Cité, 15 rue Jean-Antoine de Baïf, F-75205 Paris Cedex 13, France - Electrical and Computer Engineering, University of Alberta, Edmonton, Alberta, Canada T6G 2 V4 NRC National Institute for Nanotechnology, Edmonton, Alberta, Canada T6G 2M9ce
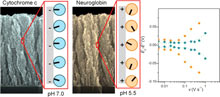
3D nanostructured transparent indium tin oxide (ITO) electrodes prepared by glancing angle deposition (GLAD) were used for the spectroelectrochemical characterization of cytochrome c (Cyt c) and neuroglobin (Nb). These small hemoproteins, involved as electron-transfer partners in the prevention of apoptosis, are oppositely charged at physiological pH and can each be adsorbed within the ITO network under different pH conditions. The resulting modified electrodes were investigated by UV-visible absorption spectroscopy coupled with cyclic voltammetry. By using nondenaturating adsorption conditions, we demonstrate that both proteins are capable of direct electron transfer to the conductive ITO surface, sharing apparent standard potentials similar to those reported in solution. Preservation of the 3D protein structure upon adsorption was confirmed by resonance Raman (rR) spectroscopy. Analysis of the derivative cyclic voltabsorptograms (DCVA) monitored either in the Soret or the Q bands at scan rates up to 1 V s-1 allowed us to investigate direct interfacial electron transfer kinetics. From the DCVA shape and scan rate dependences, we conclude that the interaction of Cyt c with the ITO surface is more specific than Nb, suggesting an oriented adsorption of Cyt c and a random adsorption of Nb on the ITO surface. At the same time, Cyt c appears more sensitive to the experimental adsorption conditions, and complete denaturation of Cyt c may occur as evidenced from cross-correlation of rR spectroscopy and spectroelectrochemistry. |

