Publication
721
Bioorg. Med. Chem. Lett. , 23 , 955-958, 2013
DOI:10.1016/j.bmcl.2012.12.057
|
|
|
|
|
|
 |
Synthesis and characterization of oligonucleotide conjugates bearing electroactive labels |
|
|
|
Julie Moreau, Nabil Dendane, Bernd Schöllhorn, Nicolas Spinelli, Claire Fave, Eric Defrancq
Institut de Topologie et de Dynamique des Systèmes (ITODYS), UMR 7086 CNRS/Université Paris Diderot, Sorbonne Paris Cité, 15 rue Jean-Antoine de Baïf, 75205 Paris Cedex, France
Département de Chimie Moléculaire, UMR CNRS 5250/Université Joseph Fourier, BP 53-38041, Grenoble Cedex 9, France
Laboratoire d’Electrochimie Moléculaire, UMR 7591 CNRS/Université Paris Diderot, Sorbonne Paris Cité, 15 rue Jean-Antoine de Baïf, 75205 Paris Cedex, France
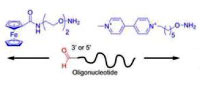
Oxime bond formation has been applied to the preparation of oligonucleotides labeled with electrochemical ferrocene and viologen labels. Aminooxy functionalized ferrocene and viologen derivatives were prepared by a straightforward route and efficiently conjugated with aldehyde containing oligonucleotides either at 3' or 5' end. Both labels were found to not disturb the recognition properties of the oligonucleotide. The versatility of the method was further demonstrated by preparing bi-functionalized conjugates with a disulfide at 3' end and an electrochemical label at 5' end. |

