Publication
723
Langmuir, 29 (17), 5360-5368, 2013
DOI: 10.1021/la401117u
|
|
|
|
|
|
 |
Switching On/Off the Chemisorption of Thioctic-Based Self-Assembled Monolayers on Gold by Applying a Moderate Cathodic/Anodic Potential |
|
|
|
Rihab Salhi, Claire Fave, Noureddine Raouafi, Khaled Boujlel, Bernd Schöllhorn and Benoît Limoges
- Laboratoire d’Electrochimie Moléculaire, UMR CNRS 7591, and Institut de Topologie et de Dynamique des Systèmes (ITODYS), UMR 7086 CNRS, Université Paris Diderot, Sorbonne Paris Cité, 15 rue Jean-Antoine de Baïf, F-75205 Paris cedex, France
- Laboratoire de Chimie Analytique et d’Electrochimie, Département de Chimie, Faculté des Sciences de Tunis, Université de Tunis El-Manar, 2092 Tunis El-Manar, Tunisia
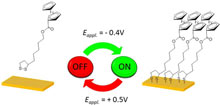
An in situ and real-time electrochemical method has been devised for quantitatively monitoring the self-assembly of a ferrocene-labeled cyclic disulfide derivative (i.e., a thioctic acid derivative) on a polycrystalline gold electrode under electrode polarization. Taking advantage of the high sensitivity, specificity, accuracy, and temporal resolution of this method, we were able to demonstrate an unexpectedly facilitated formation of the redox-active SAM when the electrode was held at a moderate cathodic potential (−0.4 V vs SCE in CH3CN), affording a saturated monolayer from only micromolar solutions in less than 10 min, and a totally impeded SAM growth when the electrode was polarized at a slightly anodic potential (+0.5 V vs SCE in CH3CN). This method literally allows for switching on/off the formation of SAMs under “soft” conditions. Moreover the cyclic disulfide-based SAM was completely desorbed at this potential contrary to the facilitated deposition of a ferrocene-labeled alkanethiol. Such a strikingly contrasting behavior could be explained by an energetically favored release of the thioctic-based SAM through homolytic cleavage of the Au–S bond followed by intramolecular cyclization of the generated thiyl diradicals. Moreover, the absence of a discernible transient faradaic current response during the potential-assisted adsorption/desorption of the redox-labeled cyclic disulfide led us to conclude in a potential-dependent reversible surface reaction where no electron is released or consumed. These results provide new insights into the formation of disulfide-based SAMs on gold but also raise some fundamental questions about the intimate mechanism involved in the facilitated adsorption/desorption of SAMs under electrode polarization. Finally, the possibility to easily and selectively address the formation/removal of thioctic-based SAMs on gold by applying a moderate cathodic/anodic potential offers another degree of freedom in tailoring their properties and in controlling their self-assembly, nanostructuration, and/or release. |

