Publication
728
J. Am. Chem. Soc. , 135 (28),10492-10502, 2013
DOI:10.1021/ja403656w
|
|
|
|
|
|
 |
Proton-electron transport and transfer in electrocatalytic films. Application to a cobalt-based O2-evolution catalyst |
|
|
|
Daniel Kwabena Bediako, Cyrille Costentin, Evan C. Jones, Daniel G. Nocera, and Jean-Michel Savéant
Department of Chemistry and Chemical Biology, 12 Oxford Street, Harvard University, Cambridge, Massachusetts 02138-2902, United States
Sorbonne Paris Cité, Laboratoire d'Electrochimie Moléculaire, Unité Mixte de Recherche Université–CNRS No. 7591, Université Paris Diderot, Bâtiment Lavoisier, 15 Rue Jean de Baïf, 75205 Paris Cedex 13, France
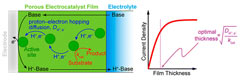
Solar-driven electrochemical transformations of small molecules, such as water splitting and CO2 reduction, pertinent to modern energy challenges, require the assistance of catalysts preferably deposited on conducting or semiconducting surfaces. Understanding mechanisms and identifying the factors that control the functioning of such systems are required for rational catalyst optimization and improved performance. A methodology is proposed, in the framework of rotating disk electrode voltammetry, to analyze the current responses expected in the case of a semigeneral reaction scheme involving a proton-coupled catalytic reaction associated with proton-coupled electron hopping through the film as rate controlling factors in the case where there is no limitation by substrate diffusion. The predictions concern the current density vs overpotential (Tafel) plots and their dependence on buffer concentration (including absence of buffer), film thickness and rotation rate. The Tafel plots may have a variety of slopes (e.g., F/RT ln 10, F/2RT ln 10, 0) that may even coexist within the overpotential range of a single plot. We show that an optimal film thickness exists beyond which the activity of the film plateaus. Application to water oxidation by films of a cobalt-based oxidic catalyst provides a successful test of the applicability of the proposed methodology, which also provides further insight into the mechanism by which these cobalt-based films catalyze the oxidation of water. The exact nature of the kinetic and thermodynamic characteristics that have been derived from the analysis is discussed as well as their use in catalyst benchmarking. |

