Publication
735
J. Am. Chem. Soc., 135 (47), 17671-17674, 2013
DOI:10.1021/ja407988w
|
|
|
|
|
|
|
|
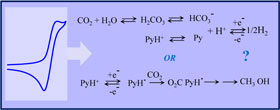
Electrochemistry of acids on platinum. Application to the reduction of carbon dioxide in the presence of pyridinium ion in water. |
|
|
|
Cyrille Costentin, Juan Carlos Canales, Baptiste Haddou, and Jean-Michel Savéant
Université Paris Diderot, Sorbonne Paris Cité, Laboratoire d’Electrochimie Moléculaire, Unité Mixte de Recherche Université-CNRS no. 7591, Bâtiment Lavoisier, 15 rue Jean de Baı̈f, 75205 Paris Cedex 13, France
A detailed cyclic voltammetric investigation of the reduction of moderately weak acids on platinum reveals that they are reduced in two steps: one involving the hydrated protons initially present at equilibrium and the second the reduction of the acid through its prior conversion into hydrated protons. The reduction of pyridinium ions (protonated pyridine) follows this reaction scheme as does any other acid of similar pK (e.g., acetic acid). Rather than being catalytically reduced, CO2 plays a similar role through its prior conversion to carbonic acid. No trace of methanol or formate could be detected upon preparative-scale electrolysis of CO2 on the same electrode in the presence of pyridinium ions. |

