Publication
738
Chem. Comm., 50, 1894-1896, 2014
DOI:10.1039/C3CC48489K
|
 PDF PDF |
|
|
|
|
|
|
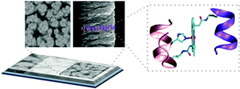
Spectroelectrochemistry of FeIII- and CoIII- mimochrome VI artificial enzymes immobilized in mesoporous ITO electrodes. |
|
|
|
Rosa Vitale, Liliana Lista, Stephanie Truong, Ryan T. Tucker, Michael Brett, Benoit Limoges, Vincenzo Pavone, Angela Lombardi, and Véronique Balland
Department of Chemical Sciences, Complesso Universitario Monte S. Angelo, University of Naples “Federico II”, Via Cintia, 80126 Naples, Italy
ITODYS, UMR CNRS 7086, Université Paris Diderot, Sorbonne Paris Cité, 15, rue Jean-Antoine de Baïf, 75205 Paris Cedex 13, France
Laboratoire de Céramique, Université de Constantine 1, Constantine, Algeria
Electrical and Computer Engineering, University of Alberta, Edmonton, Canada T6G 2V4
NRC National Institute for Nanotechnology, Edmonton, Canada T6G 2M9
Laboratoire d'Electrochimie Moléculaire, Université Paris Diderot, UMR CNRS 7591, 15, rue Jean-Antoine de Baïf, 75205 Paris Cedex 13, France
UV-visible absorption spectroelectrochemistry elucidated the different redox behaviours of FeIII- and CoIII-mimochrome VI artificial enzymes, adsorbed on mesoporous conductive films of ITO. The reduction of the ferric complex was rapid and reversible, while the cobaltic complex exhibited irreversible processes probably related to multiple coordination states. |

