Publication
741
ACS Cat., 4 (2), 681-687, 2014
DOI:10.1021/cs4011698
|
|
|
|
|
|
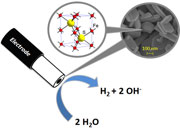 |
Bioinspired iron sulfide nanoparticles for cheap and long-lived electrocatalytic molecular hydrogen evolution in neutral water. |
|
|
|
Carlo Di Giovanni, Wei-An Wang, Sophie Nowak, Jean-Marc Grenèche, Hélène Lecoq, Ludovic Mouton, Marion Giraud, and Cédric Tard
Laboratoire d’Electrochimie Moléculaire, UMR 7591, CNRS, Université Paris Diderot – Paris 7, Bât. Lavoisier, 15 rue Jean-Antoine de Baïf, 75205 Paris Cedex 13, France
Laboratoire ITODYS, UMR 7086 CNRS, Université Paris Diderot, Sorbonne Paris Cité, 15 rue Jean-Antoine de Baïf, F-75205 Paris Cedex 13, France
Institut des Molécules et Matériaux du Mans, IMMM, UMR 6283 CNRS, Université du Maine, Avenue Olivier Messiaen, F-72085 Le Mans Cedex 9, France
Alternative materials to platinum-based catalysts are required to produce molecular hydrogen from water at low overpotentials. Transition-metal chalcogenide catalysts have attracted significant interest over the past few years because of their activity toward proton reduction and their relative abundance compared with platinum. We report the synthesis and characterization of a new type of iron sulfide (FeS, pyrrhotite) nanoparticles prepared via a solvothermal route. This material can achieve electrocatalysis for molecular hydrogen evolution with no structural decomposition or activity decrease for at least 6 days at a mild overpotential in neutral water at room temperature. |

