Publication
745
Inorg. Chem., 53 (5), 2545-2553, 2014
DOI:110.1021/ic402843y
|
|
|
|
|
|
|
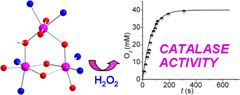
Trinuclear Manganese Complexes of Unsymmetrical Polypodal Diamino N3O3 Ligands with an Unusual [Mn3(µ-OR)4]5+ Triangular Core: Synthesis, Characterization, and Catalase Activity. |
|
|
|
Gabriela N. Ledesma, Elodie Anxolabéhère-Mallart, Eric Rivière, Sonia Mallet-Ladeira, Christelle Hureau, and Sandra R. Signorella
IQUIR (Instituto de Química Rosario), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Facultad de Ciencias Bioquímicas y Farmacéuticas, Universidad Nacional de Rosario, Suipacha 531, S2002LRK Rosario, Argentina
Laboratoire Electrochimie Moléculaire-UMR CNRS 7591, University of Paris Diderot, Sorbonne Paris Cité15 rue Jean de Baïf, 75251 Paris, Cedex 13, France
ICMMO-UMR 8182, Equipe Chimie Inorganique, Université Paris-Sud, 91405 Orsay, France
Institut de Chimie de Toulouse, FR 2599, 118 Route de Narbonne, F-31062 Toulouse, France
CNRS, LCC (Laboratoire de Chimie de Coordination) and UPS, INPT, LCC, Université de Toulouse, 205 route de Narbonne, F-31077 Toulouse, France
Two new tri-MnIII complexes of general formula [Mn3L2(µ-OH)(OAc)]ClO4 (H3L = 1-[N-(2-pyridylmethyl),N-(2-hydroxybenzyl)amino]-3-[N'-(2-hydroxybenzyl),N'-(4-X-benzyl)amino]propan-2-ol; 1ClO4, X = Me; 2ClO4, X = H) have been prepared and characterized. X-ray diffraction analysis of 1ClO4 reveals that the complex cation possesses a Mn3(µ-alkoxo)2(µ-hydroxo)(µ-phenoxo)4+ core, with the three Mn atoms bound to two fully deprotonated N3O3 chelating L3–, one exogenous acetato ligand, and one hydroxo bridge, the structure of which is retained upon dissolution in acetonitrile or methanol. The three Mn atoms occupy the vertices of a nearly isosceles triangle (Mn1···Mn3 = 3.6374(12) Å, Mn2···Mn3 3.5583(13) Å, and Mn1···Mn2 3.2400(12) Å), with one substitution-labile site on the apical Mn ion occupied by terminally bound monodentate acetate. Temperature-dependent magnetic susceptibility studies indicate the presence of predominant antiferromagnetic intramolecular interactions between MnIII ions in 1ClO4. Complexes 1ClO4 and 2ClO4 decompose H2O2 at comparable rates upon initial binding of peroxide through acetate substitution, with retention of core structure during catalysis. Kinetic and spectroscopic studies suggest that these complexes employ the [Mn–(µ-oxo/aquo)–Mn]4+ moiety to activate peroxide, with the additional (µ-alkoxo)(µ-phenoxo)Mn(µ-alkoxo) metallobridge carrying out a structural function. |

