Publication
746
Biochem. J., 459 (1), 37-45, 2014
DOI:10.1042/bj20131567
|
|
|
|
|
|
|
Substrate interaction dynamics and oxygen control in the active site of thymidylate synthase ThyX. |
|
|
|
Hubert F. Becker, Kamel Djaout, Isabelle Lamarre, Jonathan E. Ulmer, Delphine Schaming, Véronique Balland, Ursula Liebl, Hannu Myllykallio, and Marten H. Vos
Laboratory for Optics and Biosciences, CNRS Ecole Polytechnique, 91128 Palaiseau, France
INSERM U696, 91128 Palaiseau, France
UPMC Univ Paris 06, 75005 Paris, France
Laboratoire d’Electrochimie Moléculaire, UMR CNRS 7591, Université Paris Diderot, Sorbonne Paris Cité, 15, rue Jean-Antoine de Baïf, 75205 Paris Cedex 13, France
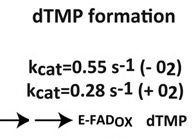
Thymidylate synthase ThyX, required for DNA synthesis in many pathogenic bacteria, is considered a promising antimicrobial target. It binds FAD and three substrates, producing dTMP (2′-deoxythymidine-5′-monophosphate) from dUMP (2′-deoxyuridine-5′-monophosphate). However, ThyX proteins also act as NADPH oxidase by reacting directly with O2. In the present study we investigated the dynamic interplay between the substrates and their role in competing with this wasteful and potentially harmful oxidase reaction in catalytically efficient ThyX from Paramecium bursaria Chlorella virus-1. dUMP binding accelerates the O2-insensitive half-reaction between NADPH and FAD by over four orders of magnitude to ~30 s−1. Thus, although dUMP does not have a direct role in FAD reduction, any turnover with molecular O2 requires its presence. Inversely, NADPH accommodation accelerates dUMP binding ~3-fold and apparently precedes dUMP binding under physiological conditions. In the oxidative half-reaction, excess CH2H4folate (N5,N10-methylene-5,6,7,8-tetrahydrofolate) was found to re-oxidize FADH2 within 1 ms, thus very efficiently competing with FADH2 oxidation by O2 (1.5 s−1 under aerobic conditions). The resulting reaction scheme points out how the interplay between the fast reactions with the native substrates, although not rate-limiting for overall catalysis, avoids NADPH oxidase activity in aerobic micro-organisms, including many pathogens. These observations also explain why ThyX proteins are also present in aerobic micro-organisms. |

