Publication
757
Energy Environ. Sci., 7 (11), 3808-3814, 2014
DOI:10.1039/C4EE01709A
|
|
|
|
|
|
|
|
Toward the rational benchmarking of homogeneous
H2-evolving catalysts |
|
|
|
Vincent Artero and Jean-Michel Savéant
Univ Grenoble Alpes, CNRS and CEA, Laboratoire de Chimie et Biologie des Métaux, 17 rue des martyrs, 38000 Grenoble, France
Université Paris Diderot, Sorbonne Paris Cité, Laboratoire d’Electrochimie Moléculaire, Unité Mixte de Recherche Université - CNRS No 7591, Bâtiment Lavoisier, 15 rue Jean de Baïf, 75205 Paris Cedex 13, France
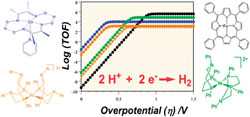
Molecular electrocatalysts for H2 evolution are usually studied under various conditions (solvent and proton sources) that prevent direct comparison of their performances. We provide here a rational method for such a benchmark based on (i) the recent analysis of the current-potential response for two-electron-two-step mechanisms and (ii) the derivation of catalytic Tafel plots reflecting the interdependency of turnover frequency and overpotential based on the intrinsic properties of the catalyst, independent of contingent factors such as cell characteristics. Such a methodology is exemplified on a series of molecular catalysts among the most efficient in the recent literature. |

