Publication
767
J. Org. Chem., 80 (5), 2733-2739, 2015
DOI:10.1021/jo502953t
|
|
|
|
|
|
 |
Photoremoval of protecting groups: mechanistic aspects of 1,3-dithiane conversion to a carbonyl group |
|
|
|
Gabriela Oksdath-Mansilla, Viviane Hajj, Diego M. Andrada, Juan E. Argüello, Julien Bonin, Marc Robert, and Alicia B. Peñéñory
INFIQC-CONICET-UNC, Dpto. de Química Orgánica, Facultad de Ciencias Químicas, Universidad Nacional de Córdoba, Ciudad Universitaria, X5000HUA Córdoba, Argentina
Laboratoire d’Electrochimie Moléculaire, Unité Mixte de Recherche Université - CNRS No 7591, Université Paris Diderot, Sorbonne Paris Cité, Bâtiment Lavoisier, 15 rue Jean Antoine de Baïf, 75205 Paris Cedex 13, France
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Strasse, D-35032 Marburg, Germany
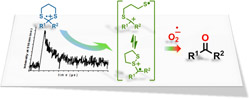
Photodeprotection of 1,3-dithianes in the presence of thiapyrylium was performed to return to the parent carbonyl compound, and the mechanism was studied by steady state photolysis, laser flash photolysis, and theoretical calculations. Electron transfer from dithianes to triplet sensitizers is extremely fast, and the decay of dithiane radical cations was not affected by the presence of water or oxygen as the consequence of a favorable unimolecular fragmentation pathway. Similar behaviors were observed for dithianes bearing electron-releasing or electron-withdrawing substituents on the aryl moiety, evidenced by C–S bond cleavage to form a distonic radical cation species. The lack of reaction under nitrogen atmosphere, requirement of oxygen for good conversion yields, inhibition of the photodeprotection process by the presence of p-benzoquinone, and absence of a labeled carbonyl final product when the reaction is performed in the presence of H218O all suggest that the superoxide anion drives the deprotection reaction. Density functional theory computational studies on the reactions with water, molecular oxygen, and the superoxide radical anion support the experimental findings. |

