Publication
772
J. Am. Chem. Soc., 137 (16), 5461-5467, 2015
DOI:10.1021/jacs.5b00914
|
|
|
|
|
|
 |
Benchmarking of Homogeneous Electrocatalysts. Overpotential, Turnover Frequency, Limiting Turnover Number |
|
|
|
Cyrille Costentin, Guillaume Passard, and Jean-Michel Savéant
Université Paris Diderot, Sorbonne Paris Cité, Laboratoire d’Electrochimie Moléculaire, Unité Mixte de Recherche Université - CNRS N° 7591, Bâtiment Lavoisier, 15 rue Jean de Baïf, 75205 Paris Cedex 13, France
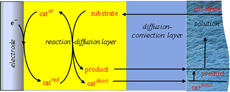
In relation to contemporary energy challenges, a number of molecular catalysts for the activation of small molecules, mainly based on transition metal complexes, have been developed. The time has thus come to develop tools allowing the benchmarking of these numerous catalysts. Two main factors of merit are addressed. One involves their intrinsic catalytic performances through the comparison of “catalytic Tafel plots” relating the turnover frequency to the overpotential independently of the characteristics of the electrochemical cell. The other examines the effect of deactivation of the catalyst during the course of electrolysis. It introduces the notion of the limiting turnover number as a second key element of catalyst benchmarking. How these two factors combine with one another to control the course of electrolysis is analyzed in detail, leading to procedures that allow their separate estimation from measurements of the current, the charge passed, and the decay of the catalyst concentration. Illustrative examples from literature data are discussed. |

