Publication
779
J. Mater. Chem. A, 3 (31), 16190-16197, 2015
DOI:10.1039/C5TA03430B
|
|
|
|
|
|
 |
Fast magnetically driven electrodeposition of amorphous metal oxides water oxidation catalysts from carbon-coated metallic nanoparticles |
|
|
|
Jing Zhu, François Lambert, Clotilde Policar, François Mavré, and Benoît Limoges
École Normale Supérieure – PSL Research University, Département de Chimie, Sorbonne Universités – UPMC Univ Paris 06, CNRS UMR 7203 LBM, 24 rue Lhomond, F-75005 Paris, France
Laboratoire d'Electrochimie Moléculaire, UMR 7591 CNRS, Université Paris Diderot, Sorbonne Paris Cité, 15 rue Jean-Antoine de Baïf, F-75205 Paris Cedex 13, France
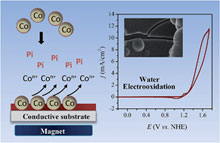
We report a new approach for efficient electrodeposition of amorphous metal oxide water oxidation catalysts on an electrode surface. A catalytic metal-based film was obtained by means of anodic oxidation of metallic nanoparticles, namely carbon-coated cobalt nanoparticles or carbon-coated nickel nanoparticles. Interestingly, these particles are intrinsically conductive and possess magnetic properties which make it easy to collect them on an electrode surface using a simple magnet to form a porous conductive particulate film. Upon anodic polarization in an appropriate electrolyte, the particulate film is rapidly converted into an amorphous metal-based catalytic film that efficiently catalyzes the oxidation of water at neutral pH. Compared to Nocera's method based on anodic electrodeposition of a metal salt in solution, this new electrodeposition strategy offers the key advantage of supplying metal ions in a solid and metallic form, leading to a fast release of high local concentrations of metal ions right at the spot of the film formation (i.e., in the vicinity of the electrode surface). This plays a decisive role in the formation rate of the catalytic film, allowing the deposition of the oxygen-evolving catalyst in a remarkably short-time. Moreover, the methodology can be easily extended to a wide range of metal particles of different nature and sizes, and also to their mixtures, finally offering a new degree of flexibility and opportunities not only in the preparation of metal-based water oxidation catalysts, but also in the preparation of inorganic metal-based catalysts for hydrogen or oxygen evolution. |

