Publication
780
J. Am. Chem. Soc., 137 (34), 10918-10921, 2015
DOI:10.1021/jacs.5b06535
|
|
|
|
|
|
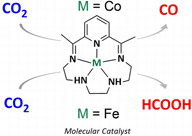 |
Molecular catalysis of the electrochemical and photochemical reduction of CO2 with earth abundant metal complexes. Selective production of CO vs HCOOH by switching of the metal center |
|
|
|
Lingjing Chen, Zhenguo Guo, Xi-Guang Wei, Charlotte Gallenkamp, Julien Bonin, Elodie Anxolabéhère-Mallart, Kai-Chung Lau, Tai-Chu Lau, and Marc Robert
Department of Biology and Chemistry, Institute of Molecular Functional Materials, City University of Hong Kong, Tat Chee Avenue, Kowloon, Hong Kong SAR, China
Université Paris Diderot, Sorbonne Paris Cité, Laboratoire d’Electrochimie Moléculaire, UMR 7591 CNRS, 15 rue Jean-Antoine de Baïf, F-75205 Paris Cedex 13, France
Molecular catalysis of carbon dioxide reduction using earth-abundant metal complexes as catalysts is a key challenge related to the production of useful products—the “solar fuels”—in which solar energy would be stored. A direct approach using sunlight energy as well as an indirect approach where sunlight is first converted into electricity could be used. A CoII complex and a FeIII complex, both bearing the same pentadentate N5 ligand (2,13-dimethyl-3,6,9,12,18-pentaazabicyclo[12.3.1]octadeca-1(18),2,12,14,16-pentaene), were synthesized, and their catalytic activity toward CO2 reduction was investigated. Carbon monoxide was formed with the cobalt complex, while formic acid was obtained with the iron-based catalyst, thus showing that the catalysis product can be switched by changing the metal center. Selective CO2 reduction occurs under electrochemical conditions as well as photochemical conditions when using a photosensitizer under visible light excitation (? > 460 nm, solvent acetonitrile) with the Co catalyst. In the case of the Fe catalyst, selective HCOOH production occurs at low overpotential. Sustained catalytic activity over long periods of time and high turnover numbers were observed in both cases. A catalytic mechanism is suggested on the basis of experimental results and preliminary quantum chemistry calculations. |

