Publication
783
J. Am. Chem. Soc., 137 (42), 13535-13544, 2015
DOI:10.1021/jacs.5b06834
|
|
|
|
|
|
 |
Molecular catalysis of O2 reduction by iron porphyrins in water. Heterogeneous vs. homogeneous pathways |
|
|
|
Cyrille Costentin, Hachem Dridi, and Jean-Michel Savéant
Sorbonne Paris Cité, Laboratoire d’Electrochimie Moléculaire, Unité Mixte de Recherche Université − CNRS No. 7591, Université Paris Diderot, Bâtiment Lavoisier, 15 rue Jean de Baïf, 75205 Cedex 13 Paris, France
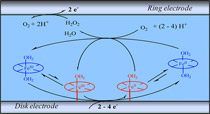
Despite decades of active attention, important problems remain pending in the catalysis of dioxygen reduction by iron porphyrins in water in terms of selectivity and mechanisms. This is what happens, for example, for the distinction between heterogeneous and homogeneous catalysis for soluble porphyrins, for the estimation of H2O2/H2O product selectivity, and for the determination of the reaction mechanism in the two situations. With water-soluble iron tetrakis(N-methyl-4-pyridyl)porphyrin as an example, procedures are described that allow one to operate this distinction and determine the H2O2/H2O product ratio in each case separately. It is noteworthy that, despite the weak adsorption of the iron(II) porphyrin on the glassy carbon electrode, the contribution of the adsorbed complex to catalysis rivals that of its solution counterpart. Depending on the electrode potential, two successive catalytic pathways have been identified and characterized in terms of current–potential responses and H2O2/H2O selectivity. These observations are interpreted in the framework of the commonly accepted mechanism for catalytic reduction of dioxygen by iron porphyrins, after checking its compatibility with a change of oxygen concentration and pH. The difference in intrinsic catalytic reactivity between the catalyst in the adsorbed state and in solution is also discussed. The role of heterogeneous catalysis with iron tetrakis(N-methyl-4-pyridyl)porphyrin has been overlooked in previous studies because of its water solubility. The main objective of the present contribution is therefore to call attention, by means of this emblematic example, to such possibilities to reach a correct identification of the catalyst, its performances, and reaction mechanism. This is a question of general interest, so that reduction of dioxygen remains a topic of high importance in the context of contemporary energy challenges. |

