Publication
791
J. Am. Chem. Soc., 138 (8), 2492-2495, 2016
DOI:10.1021/jacs.5b12652
|
|
|
|
|
|
 |
Noncovalent immobilization of a molecular iron-based electrocatalyst on carbon electrodes for selective, efficient CO2-to-CO conversion in water |
|
|
|
Antoine Maurin and Marc Robert
Laboratoire d’Electrochimie Moléculaire, Univerity of Paris Diderot, Sorbonne Paris Cité, UMR 7591 CNRS, 15 rue Jean- Antoine de Baïf, F-75205 Paris Cedex 13, France
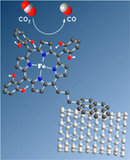
Catalysis of fuel-producing reactions can be transferred from homogeneous solution to surface via attachment of the molecular catalyst. A pyrene-appended iron triphenyl porphyrin bearing six pendant OH groups on the phenyl rings in all ortho and ortho' positions was immobilized on carbon nanotubes via noncovalent interactions and further deposited on glassy carbon. X-ray photoelectron spectroscopy and electrochemistry confirm catalyst immobilization. Using the carbon material, highly selective and rapid catalysis of the reduction of CO2 into CO occurs in water (pH 7.3) with 480 mV overpotential. Catalysis could be sustained for hours without loss of activity and selectivity, and high turnover number was obtained. |

