Publication
792
ACS Catal., 6 (4), 2626–2631, 2016
DOI:10.1021/acscatal.5b02443
|
|
|
|
|
|
 |
Low-cost nanostructured iron sulfide electrocatalysts for PEM water electrolysis |
|
|
|
Carlo Di Giovanni, Álvaro Reyes-Carmona, Anaïs Coursier, Sophie Nowak, Jean?Marc Grenèche, Hélène Lecoq, Ludovic Mouton, Jacques Rozière, Deborah Jones, Jennifer Peron, Marion Giraud, and Cédric Tard
Laboratoire d’Électrochimie Moléculaire, UMR 7591 CNRS, Université Paris Diderot, Sorbonne Paris Cité, 15 rue Jean-Antoine de Baı̈f, F-75205 Paris Cedex 13, France
Institut Charles Gerhardt, UMR 5253, Aggregates, Interfaces and Materials for Energy, Université de Montpellier, Place Eugène Bataillon, F-34095 Montpellier Cedex 5, France
Laboratoire ITODYS, UMR 7086 CNRS, Université Paris Diderot, Sorbonne Paris Cité, 15 rue Jean-Antoine de Baı̈f, F-75205 Paris Cedex 13, France
Institut des Molécules et Matériaux du Mans, IMMM, UMR 6283 CNRS, Université du Maine, Avenue Olivier Messiaen, F-72085 Le Mans Cedex 9, France
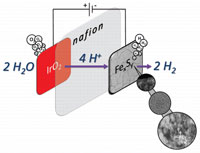
In the context of increased application of proton exchange membrane electrolyzers, the development of cheap and long-lived transition-metal hydrogen evolution reaction electrocatalysts is required to circumvent nonsustainable platinum-based electrocatalysts. Herein we report the synthesis and characterization of robust iron–sulfide nanoparticles from low-cost precursors (i.e., FeCl3 and thiourea) using an easily scalable soft synthesis technique, which can achieve electrocatalysis. In the series of nanoparticles studied, we show that pyrite FeS2 is the most active, in comparison with greigite Fe3S4 and pyrrhotite Fe9S10, in a three-electrode electrochemical cell, the electrocatalysis starting at an overpotential of ~180 mV. These three materials exhibit a very stable behavior during the catalysis, with no activity decrease for at least 5 days. FexSy catalysts have been tested in a PEM electrolysis single cell, and pyrite FeS2 allows achievement of a current density of 2 A/cm2 at a voltage of 2.3 V. |

