Publication
794
Chem.-As. J., 11 (8), 1281–1287, 2016
DOI:10.1002/asia.201501446
|
|
|
|
|
|
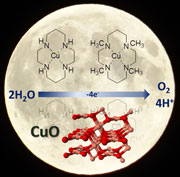 |
Heterogeneous and homogeneous routes in water oxidation catalysis starting from Cu(II) complexes with tetraaza macrocyclic ligands |
|
|
|
Andrea Sartorel, Andrea Prevedello, Irene Bazzan, Nicola Dalle Carbonare, Angela Giuliani, Sunil Bhardwaj, Cristina Africh, Cinzia Cepek, Roberto Argazzi, Marcella Bonchio, Stefano Caramori, and Marc Robert
Department of Chemical Sciences, University of Padova and Institute on Membrane Technology, Padova, Italy
Department of Chemical and Pharmaceutical Sciences, University of Ferrara, Ferrara, Italy
TASC-INFM National Laboratory, Basovizza, TS, Italy
ISOF-CNR c/o Dept. of Chemical and Pharmaceutical Sciences, University of Ferrara, Ferrara, Italy
Université Paris Diderot, Sorbonne Paris Cité, Laboratoire d'Electrochimie Moléculaire, UMR CNRS N° 7591, Bâtiment Lavoisier, Paris Cedex 13, France
Since the first report in 2012, molecular copper complexes have been proposed as efficient electrocatalysts for water oxidation reactions, carried out in alkaline/neutral aqueous media. However, in some cases the copper species have been recognized as precursors of an active copper oxide layer, electrodeposited onto the working electrode. Therefore, the question whether copper catalysis is molecular or not is particularly relevant in the field of water oxidation. In this study, we investigate the electrochemical activity of copper(II) complexes with two tetraaza macrocyclic ligands, distinguishing heterogeneous or homogeneous processes depending on the reaction media. In an alkaline aqueous solution, and upon application of an anodic bias to working electrodes, an active copper oxide layer is observed to electrodeposit at the electrode surface. Conversely, water oxidation in neutral aqueous buffers is not associated to formation of the copper oxide layer, and could be exploited to evaluate and optimize a molecular, homogeneous catalysis. |

