Publication
795
J. Am. Chem. Soc., 138 (17), 5615–5622, 2016
DOI:10.1021/jacs.6b00737
|
|
|
|
|
|
 |
Conduction and reactivity in heterogeneous-molecular catalysis. New insights in water oxidation catalysis by phosphate cobalt oxide films |
|
|
|
Cyrille Costentin, Thomas R. Porter, and Jean-Michel Savéant
Université Paris Diderot, Sorbonne Paris Cité, Laboratoire d’Electrochimie Moléculaire, Unité Mixte de Recherche Université−CNRS No. 7591, Bâtiment Lavoisier, 15 rue Jean de Baïf, 75205 Paris Cedex 13, France
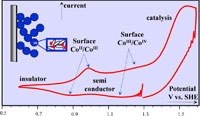
Cyclic voltammetry of phosphate cobalt oxide (CoPi) films catalyzing O2-evolution from water oxidation as a function of scan rate, phosphate concentration and film thickness allowed for new insights into the coupling between charge transport and catalysis. At pH = 7 and low buffer concentrations, the film is insulating below 0.8 (V vs SHE) but becomes conductive above 0.9 (V vs SHE). Between 1.0 to 1.3 (V vs SHE), the mesoporous structure of the film gives rise to a large thickness-dependent capacitance. At higher buffer concentrations, two reversible proton-coupled redox couples appear over the capacitive response with 0.94 and 1.19 (V vs SHE) pH = 7 standard potentials. The latter is, at most, very weakly catalytic and not responsible for the large catalytic current observed at higher potentials. CV-response analysis showed that the amount of redox-active cobalt-species in the film is small, less than 10% of total. The catalytic process involves a further proton-coupled-electron-transfer and is so fast that it is controlled by diffusion of phosphate, the catalyst cofactor. CV-analysis with newly derived relationships led to a combination of the catalyst standard potential with the catalytic rate constant and a lower-limit estimation of these parameters. The large currents resulting from the fast catalytic reaction result in significant potential losses related to charge transport through the film. CoPi films appear to combine molecular catalysis with semiconductor-type charge transport. This mode of heterogeneous molecular catalysis is likely to occur in many other catalytic films. |

