Publication
796
Proc. Natl. Acad. Sci. U.S.A., 113 (20) 5526-5529, 2016
DOI:10.1073/pnas.1604628113
|
|
|
|
|
|
 |
Efficient electrolyzer for CO2 splitting in neutral water using earth-abundant materials |
|
|
|
Arnaud Tatin, Clément Comminges, Boniface Kokoh, Cyrille Costentin, Marc Robert, and Jean-Michel Savéant
Laboratoire d'Electrochimie Moléculaire, Unité Mixte de Recherche Université - CNRS No 7591, Université Paris Diderot, Sorbonne Paris Cité, 75205 Paris Cedex 13, France
Unité Mixte de Recherche Université - CNRS No 7285, Université de Poitiers, 86022 Poitiers Cedex, France
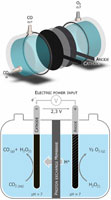
Low-cost, efficient CO2-to-CO+O2 electrochemical splitting is a key step for liquid-fuel production for renewable energy storage and use of CO2 as a feedstock for chemicals. Heterogeneous catalysts for cathodic CO2-to-CO associated with an O2-evolving anodic reaction in high-energy-efficiency cells are not yet available. An iron porphyrin immobilized into a conductive
Nafion/carbon powder layer is a stable cathode producing CO in pH neutral water with 90% faradaic efficiency. It is coupled
with a water oxidation phosphate cobalt oxide anode in a home-made electrolyzer by means of a Nafion membrane. Current densities
of approximately 1 mA/cm2 over 30-h electrolysis are achieved at a 2.5-V cell voltage, splitting CO2 and H2O into CO and O2 with a 50% energy efficiency. Remarkably, CO2 reduction outweighs the concurrent water reduction. The setup does not prevent high-efficiency proton transport through the to set up an efficient, low-voltage, electrochemical cell that converts CO2 into CO and O2 by associating a cathodic-supported molecular catalyst based on an abundant transition metal with a cheap, easy-to-prepare anodic catalyst oxidizing water into O2. |

