Publication
797
J. Power Sources, 324, 253-260, 2016
DOI:10.1016/jpowsour.2016.05.095
|
|
|
|
|
|
 |
Highly efficient photocatalytic hydrogen evolution from nickel quinolinethiolate complexes under visible light irradiation |
|
|
|
Heng Rao, Wen-Qian Yu, Hui-Qin Zheng, Julien Bonin, Yao-Ting Fan, and Hong-Wei Hou
College of Chemistry and Molecular Engineering, Zhengzhou University, Zhengzhou, 450001, PR China
Université Paris Diderot, Sorbonne Paris Cité, Laboratoire d’Electrochimie Moléculaire, UMR 7591 CNRS, 15 rue Jean-Antoine de Baïf, F-75205, Paris Cedex 13, France
School of Chemistry and Chemical Engineering, Sun Yat-sen University, Guangzhou, 510275, PR China
Department of Chemistry, Henan Institute of Education, Zhengzhou, 450046, PR China
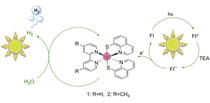
Earth-abundant metal complexes have emerged as promising surrogates of platinum for catalyzing the hydrogen evolution reaction (HER). In this study, we report the design and synthesis of two novel nickel quinolinethiolate complexes, namely [Ni(Hqt))2 (4, 4′-Z-2, 2′-bpy)] (Hqt = 8-quinolinethiol, Z = — H [1] or —CH3 [2], bpy = bipyridine). An efficient three-component photocatalytic homogeneous system for hydrogen generation working under visible light irradiation was constructed by using the target complexes as catalysts, triethylamine (TEA) as sacrificial electron donor and xanthene dyes as photosensitizer. We obtain turnover numbers (TON, vs. catalyst) for H2 evolution of 5923/7634 under the optimal conditions with 5.0 × 10−6 M complex 1/2 respectively, 1.0 × 10−3 M fluorescein and 5% (v/v) TEA at pH 12.3 in EtOH/H2O (1:1, v/v) mixture after 8 h irradiation (λ > 420 nm). We discuss the mechanism of H2 evolution in the homogeneous photocatalytic system based on fluorescence spectrum and cyclic voltammetry data. |

