Publication
807
Proc. Natl. Acad. Sci. U.S.A., 113 (42) 11756-11758, 2016
DOI:10.1073/pnas.1613406113
|
|
|
|
|
|
 |
Catalysis at the nanoscale may change selectivity |
|
|
|
Cyrille Costentin and Jean-Michel Savéant
Laboratoire d’Electrochimie Moléculaire, Université Paris Diderot, Bâtiment Lavoisier, Sorbonne Paris Cité, Unité Mixte de Recherche Université–CNRS 7591, 75205 Paris Cedex 13, France
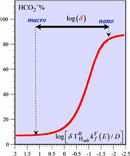
Among the many virtues ascribed to catalytic nanoparticles, the prospect that the passage from the macro- to the nanoscale may change product selectivity attracts increasing attention. To date, why such effects may exist lacks explanation. Guided by recent experimental reports, we propose that the effects may result from the coupling between the chemical steps in which the reactant, intermediates, and products are involved and transport of these species toward the catalytic surface. Considering as a thought experiment the competitive formation of hydrogen and formate upon reduction of hydrogenocarbonate ions on metals like palladium or platinum, a model is developed that allows one to identify the governing parameters and predict the effect of nanoscaling on selectivity. The model leads to a master equation relating product selectivity and thickness of the diffusion layer. The latter parameter varies considerably upon passing from the macro- to the nanoscale, thus predicting considerable variations of product selectivity. These are subtle effects in the sense that the same mechanism might exhibit a reverse variation of the selectivity if the set of parameter values were different. An expression is given that allows one to predict the direction of the effect. There has been a tendency to assign the catalytic effects of nanoscaling to chemical reactivity changes of the active surface. Such factors might be important in some circumstances. We, however, insist on the likely role of short-distance transport on product selectivity, which could have been thought, at first sight, as the exclusive domain of chemical factors. |

