Publication
811
Inorg. Chem., 55 (23), 12204-12210, 2016
DOI:10.1021/acs.inorgchem.6b01804
|
|
|
|
|
|
 |
Characterization and subsequent reactivity of an Fe-peroxo porphyrin generated by electrochemical reductive activation of O2 |
|
|
|
Raquel Oliveira, Wiem Zouari, Christian Herrero, Frédéric Banse, Bernd Schöllhorn, Claire Fave, and Elodie Anxolabéhère-Mallart
Laboratoire d’Electrochimie Moléculaire, Université Paris Diderot, Université Sorbonne Paris Cité, UMR CNRS 7591, 75205 PARIS Cedex 13, France
Institut de Chimie Moléculaire et des Matériaux d’Orsay, Université Paris Sud, Université Paris Saclay, UMR CNRS 8182, 91405 Orsay Cedex, France
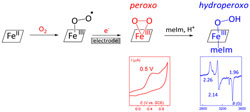
Reductive activation of O2 is achieved by using the [FeIII(F20TPP)Cl] porphyrin through electrochemical reduction of the [FeIII(F20TPP)(O2·-)] superoxo complex. Formation of the [FeIII(F20TPP) (OO)]- peroxo species is monitored by using low-temperature electronic absorption spectroscopy, electron paramagnetic resonance, and cyclic voltammetry. Its subsequent protonation to yield the [FeIII(F20TPP) (OOH)] hydroperoxo intermediate is probed using low-temperature electronic absorption spectroscopy and electron paramagnetic resonance. |

