Publication
823
Small, 13, 201603163, 2017
DOI:10.1002/smll.201603163
|
|
|
|
|
|
 |
Scaffolding of Enzymes on Virus Nanoarrays: Effects of Confinement and Virus Organization on Biocatalysis |
|
|
|
Anisha N. Patel, Agnès Anne, Arnaud Chovin, Christophe Demaille, Eric Grelet, Thierry Michon and Cécilia Taofifenua
Laboratoire d'Electrochimie Moléculaire, UMR 7591 CNRS, Université Paris Diderot, Sorbonne Paris Cité, Paris Cedex 13, France
Biologie du Fruit et Pathologie, UMR 1332 INRA, Université de Bordeaux, Villenave d'Ornon Cedex, France
Centre de Recherche Paul-Pascal, UPR 8641 CNRS, Université de Bordeaux, Pessac, France
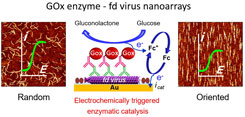
Organizing active enzyme molecules on nanometer-sized scaffolds is a promising strategy for designing highly efficient supported catalytic systems for biosynthetic and sensing applications. This is achieved by designing model nanoscale enzymatic platforms followed by thorough analysis of the catalytic activity. Herein, the virus fd bacteriophage is considered as an enzyme nanocarrier to study the scaffolding effects on enzymatic activity. Nanoarrays of randomly oriented, or directionally patterned, fd bacteriophage virus are functionalized with the enzyme glucose oxidase (GOx), using an immunological assembly strategy, directly on a gold electrode support. The scaffolding process on the virus capsid is monitored in situ by AFM (atomic force microscopy) imaging, while cyclic voltammetry is used to interrogate the catalytic activity of the resulting functional GOx-fd nanoarrays. Kinetic analysis reveals the ability to modulate the activity of GOx via nanocarrier patterning. The results evidence, for the first time, enhancement of the enzymatic activity due to scaffolding on a filamentous viral particle. |

