Publication
825
J. Chem. Crystallogr., 47 (1), 40-47, 2017
DOI:10.1007/s10870-017-0678-y
|
|
|
|
|
|
 |
Crystal Structure and Corrosion Inhibition Properties of Ferrocenyl- and Phenylendiamine-Iminomethylphenoxy Cobalt Complexes
|
|
|
|
Nadra Belhadj, Ali Ourari, Saida Keraghel, Bernd Schöllhorn, Dominique Matt
Laboratoire d’Electrochimie, d’Ingénierie Moléculaire et de Catalyse Rédox-LEIMCR, Faculté des Sciences de l’Ingenieur, Université Ferhat ABBAS, Sétif, Algeria
Département de génie des procédés, Faculté de Technologie, Université A.Mira Bejaia, Algeria
Laboratoire d’Electrochimie Moléculaire, UMR CNRS 7591, Université Paris Diderot, Paris Cedex 13, France
Laboratoire de chimie Inorganique Moléculaire et Catalyse, Institut de Chimie de Strasbourg, UMR CNRS 7177, Université de Strasbourg, Strasbourg Cedex, France
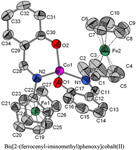
The crystal structures of bis[2-(ferrocenyl-iminomethyl)phenoxy]cobalt(II) (CoII(L)2) [monoclinic, a = 12.5466(3) Å, b = 10.6782(3) Å, c = 21.2695(6) Å, α = γ = 90°, β = 92.944(2)°, V = 2845.82(13) Å3, Z = 4, space group P21/c] and bis(2-[(4-dimethylamino-phenylimino)-methyl]-phenoxy) cobalt (II) (CoII(M)2) [triclinic, a = 10.2916(4) Å, b = 16.4867(10) Å, c = 17.6782(11) Å, α = 114.754(2)°, β = 96.614(4)°, γ = 97.736(4), Z = 4, space group P-1] dominated by extensive hydrogen bonding such as O–H···N, N–H···O and N–H···N interactions. In both structures the central CoII is displaying a slightly distorted tetrahedral coordination sphere involving two iminoethyl-phenoxy ligands. The inhibition efficiency of the Co complexes concerning the corrosion of mild steel in acidic solution has been investigated by electrochemical impedance spectroscopy. |

