Publication
832
Mol. Plant Microbe Interact., 30 (9), 754-762, 2017
DOI:10.1094/MPMI-04-17-0091-R
|
|
|
|
|
|
 |
The Potyvirus Particle Recruits the Plant Translation Initiation Factor eIF4E by Means of the VPg Covalently Linked to the Viral RNA
|
|
|
|
Geneviève Tavert-Roudet, Agnès Anne, Amandine Barra, Arnaud Chovin, Christophe Demaille, and Thierry Michon
UMR 1332 BFP, INRA, Université Bordeaux, 33883 Villenave d’Ornon, France
Laboratoire d’Electrochimie Moléculaire, UMR 7591 CNRS, Université Paris Diderot, Sorbonne Paris Cité, 15 rue Jean-Antoine de Baïf, F-75205 Paris Cedex 13, France
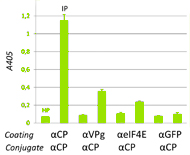
The viral protein genome-linked (VPg) of potyviruses is a protein covalently linked to the 5'- end of viral RNA. It interacts with eIF4E, a component of the cellular translation initiation complex. It has been suggested that the 5'-RNA-linked VPg could mimic the cellular mRNA cap, promoting synthesis of viral proteins. Here, we report evidence for recruitment of the plant eIF4E by Lettuce mosaic virus (LMV, potyvirus) particles via the 5'-RNA-linked VPg. Analysis of the viral population was performed by enzyme-linked immunosorbent assay-based tests, either with crude extracts of LMV-infected tissues or purified viral particles. In both cases, LMV-VPg and LMV-eIF4E subpopulations could be detected. After reaching a maximum within the first 2 weeks postinoculation, these populations decreased and very few labeled particles were found later than 3 weeks postinoculation. The central domain of VPg (CD-VPg) was found to be exposed at the surface of the particles. Using a purified recombinant lettuce eIF4E and CD-VPg-specific antibodies, we demonstrate that the plant factor binds to the VPg via its central domain. Moreover, the plant eIF4E factor could be imaged at one end of the particles purified from LMV plant extracts, by immunoredox atomic force microscopy coupled to scanning electrochemical microscopy. We discuss the biological significance of these results. |

